What is the rate constant for this reaction? In other words, a is the
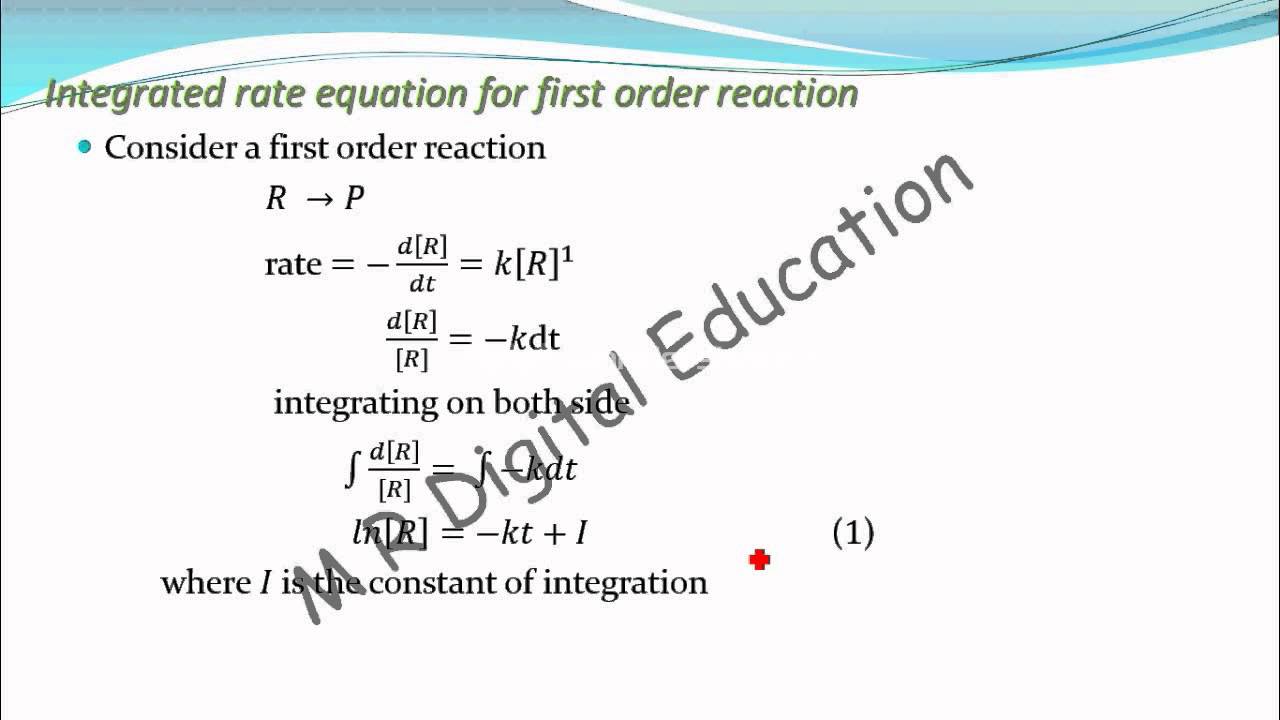
Derive The Integrated Rate Law For First Order Reaction. Consider first order reaction, a → b + c. The above equation is known as integrated rate equation for zero order reactions.
 Reaction From slideshare.net
Reaction From slideshare.net
What is the rate constant for this reaction? Asked oct 30 in chemistry by indranijain ( 35.6k points) chemical kinetics Ln[ ]=− g p+ln[ ]0
Reaction
The rate of reaction is given by dx/dt. Rate = d x d t = k 1 ( r − x).…. Consider first order reaction, a → b + c. The above equation is known as integrated rate equation for zero order reactions.
 Source: slideshare.net
Source: slideshare.net
Asked oct 30 in chemistry by indranijain ( 35.6k points) chemical kinetics Consider first order reaction, a → b + c. Suppose [a] t is the concentration of a at time = t The differential rate law is given by. 1 → 2 → (85) where k1 and k2 are the rate constants for the first and second steps, respectively.
 Source: slideserve.com
Source: slideserve.com
Consider first order reaction, a → b + c. Asked oct 30 in chemistry by indranijain ( 35.6k points) chemical kinetics 1 → 2 → (85) where k1 and k2 are the rate constants for the first and second steps, respectively. Consider the reaction r→p again. Rate = −(d[a])/dt = k[a].(1) where, [a] is the concentration of reactant at time.
 Source: schools.aglasem.com
Source: schools.aglasem.com
Consider the reaction r→p again. The differential rate law is given by. In this type of reaction, the sum of the powers of concentrations of reactants in rate law is equal to 1, that is the rate of the reaction is proportional to the first power of the concentration of the reactant. 1 → 2 → (85) where k1 and.
 Source: slideserve.com
Source: slideserve.com
Rate = −(d[a])/dt = k[a].(1) where, [a] is the concentration of reactant at time t. Consider first order reaction, a → b + c. The differential rate law is given by. Write an integrated rate law for the reaction that is first order in a and first order in (8) [1] = k[a|| plot a graph that would confirm that.
 Source: tessshebaylo.com
Source: tessshebaylo.com
Therefore, the rate law for this reaction is, rate is directly proportional to [r] we know that [r]=−kt+[r] 0. Ln[ ]=− g p+ln[ ]0 The differential rate law is given by. The equations which are obtained by integrating the differential rate laws and which gives the direct relationship between the concentrations of the reactants and time is called integrated rate.
 Source: slideserve.com
Source: slideserve.com
The equations which are obtained by integrating the differential rate laws and which gives the direct relationship between the concentrations of the reactants and time is called integrated rate laws. Integrated rate law first order reaction you derive an equation for constant the tessshlo half life of a derivation chemical kinetics cbse class 12 molecularity and zero offered by unacademy.
 Source: bartleby.com
Source: bartleby.com
Rate = −(d[a])/dt = k[a].(1) where, [a] is the concentration of reactant at time t. Suppose [a] t is the concentration of a at time = t The equations which are obtained by integrating the differential rate laws and which gives the direct relationship between the concentrations of the reactants and time is called integrated rate laws. Ln[ ]=− g.





